Why academics of investigator-initiated studies should consider outsourcing to CROs
Research driven by academics, the so-called investigator-initiated studies, are clinical studies initiated and managed by a non-pharmaceutical company researcher (e.g., individual investigators, institutions, collaborative study groups or cooperative groups) (1). In the USA, the investigator-initiated studies funded by the NIH, academic medical centers or voluntary specialty groups (charities) are classified as independent studies as these groups are not driven by money to achieve a successful outcome. These types of trials are viewed the gold standard for data and more likely to be in the best interest of the patient.
They include (1) clinical interventional studies (Phase I to IV) of approved and investigational uses of market medications or medical devices or those still in development and (2) clinical non-interventional / observational studies of real-world evidence.
The academics are responsible for the legal and regulatory responsibilities of the trial sponsor for the conduct and management of the study as defined by all applicable laws and regulations.
Investigator-initiated studies are not uncommon
Due to the high price tag for new drugs, the clinical trials are commonly funded by pharmaceutical companies. In the USA, the industry’s share of total biomedical research increased from nearly a third in 1980 to nearly two-thirds in 2000 (2). However, one analysis of funding source distribution of 119,840 drug trials registered with clinicaltrials.gov (2005–2017) showed that the proportion of studies funded by organizations other than industry or NIH has also increased (Figure 1) — mainly universities, hospitals, and other academic and non-profit agencies, classified here as “other.” Overall, these institutions and NIH funded 47.7% of all registered pharmaceutical trials, while the industry funded 52.2% of registered studies (big pharma 31.0%; small pharma 21.2%) (3).
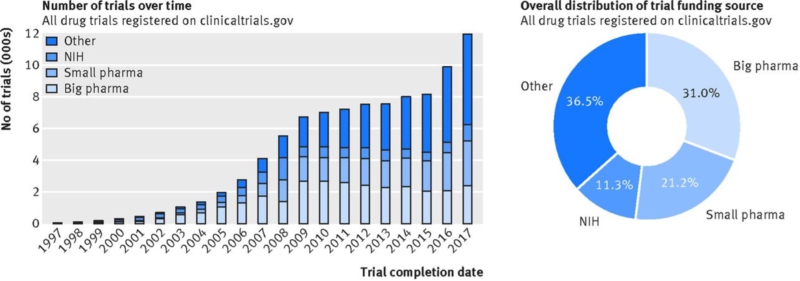
Figure 1: Funding source distribution for all trials registered with clinicaltrials.gov and conducted after 1 Jan 1997 until 19 July 2017 (based on the start date data field).
Benefits of investigator-initiated studies
The bias of industry sponsored trials cannot be overlooked. One JAMA report of integrated data from over 1,100 studies showed that industry-sponsored clinical trials are “significantly more likely to reach conclusions that were favorable to the sponsor than were non-industry studies”, possibly because of publication bias or selection of an inappropriate comparator to the drug being evaluated (Figure 2) (2).
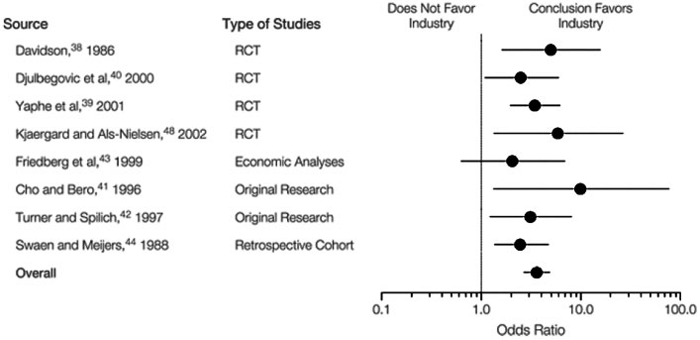
Figure 2: Relation between industry sponsorship and study outcome in original research studies.
The investigator-initiated studies are more impartial. Within academic institutions, the industry’s involvement of activities of investigator-initiated studies (e.g., protocol writing, trial conduct, data analyses and publication) can be minimized / avoided as much as possible (4). Even for those studies funded by industry but initiated by the investigators, they typically have lower overhead costs, which tend to restrict the industry sponsors to ensuring their adherence to good clinical-practice guidelines and to monitoring the data for serious adverse events, errors, and omissions. Therefore, the potential influences from the industry on the conduct of those studies can be also minimized.
As the investigator-initiated studies are largely driven by questions that generally arise beyond the completion of Phase III studies that have not been studied during Phases I–III of drug development (1). Therefore, other benefits of investigator-initiated studies include (1) data generated in a real-world setting, (2) direct application to the local population of the investigation site, and (3) providing answers to questions which are not of interest or commercially viable for the industry.
Challenges of investigator-initiated studies
For a broad perspective, investigator-initiated studies offer clinicians an opportunity to do all relevant activities of the studies, including study design, data analysis, interpretation, and data archiving. However, initiating, sustaining, and taking an investigator-initiated study to realization is not easy. While the benefits of doing an investigator-initiated study are manifold, numerous challenges need to be addressed before embarking on an investigator-initiated study. These challenges exist, ranging from regulatory submissions, continuous monitoring, to personnel training, statistics expertise, data management, and medical writing.
During the protocol development phase, the potential difficulties include inadequate / incomplete literature searching while formulating a research question, inadequate training to address ethical issues, lack of familiarity with basic research methodology, apathy toward registering the study with a clinical trials registry, and inadequate planning of safety monitoring.
During the study phase, the potential difficulties include inadequate implementation of methods, nonavailability of an internal monitor and quality control, inadequate measure to prevent dropouts, inadequate archiving facility, inadequate manpower to handle large data and execute statistical analysis, and failure to update the study report.
During the post-study phase, the possible difficulties include lack of publication policy at the protocol development state, authorship disputes for multicentric studies, dispute over data ownership, and lack of capability for publication.
The benefits of using a CRO
The most distinct benefit of outsourcing tasks to a CRO is having another professional entity conducting part of or all research activities of the studies (5). Qualified CROs will already have the established staff in place, experience / equipment needed, and vital resources prepared. It likely saves the institution time and money to establish their own personal and facilities starting from zero. Fast timeliness without time delays is another significant benefit of CROs, because they are equipped and prepared to initiate the work as soon as the contract is signed.
International registration and marketing of medication or medical device is difficult. If the drug or medical device being developed is anticipated to be approved internationally, it will be greatly beneficial to outsource to a CRO that has international experience with the approval process in each authority. The CRO with a data-driven, reliable and global regulatory affairs team can help you to meet your targets.
Choosing the right CRO is key
For any academic institutions or biotech start-ups, the essentials of outsourcing are the same. The first thing, that must be decided, is whether to work with a large full-service CRO or smaller more specialized CROs (6). For investigator-initiated studies or start-ups, there may be a remarkable risk in taking the “easy option” and trusting that a big CRO’s extensive global resources and capabilities provide assurance of successful study conduct. It is not saying that all large CROs are not committed to working with academic institutions or start-ups, however, well defined and developed needs and expectations must be understood before making the decision.
Conclusion
ICRC-Weyer GmbH, as an integrated all-phase CRO with a team of multidisciplinary specialists, has extensive experience in clinical and medical device studies (including but not limited to investigator-imitated studies), from study setup and conduct to data handling, analysis and reporting to all aspects of drug safety with all study phases and subsequently with post-marketing surveillance trials. All services can be contracted as a full-service package or as functional services, based on each customer’s needs.
Liang Gao
References
- SUVARNA V. INVESTIGATOR INITIATED TRIALS (IITS). PERSPECT CLIN RES. 2012;3(4):119–21.
- BEKELMAN JE, LI Y, GROSS CP. SCOPE AND IMPACT OF FINANCIAL CONFLICTS OF INTEREST IN BIOMEDICAL RESEARCH: A SYSTEMATIC REVIEW. JAMA. 2003;289(4):454–65.
- ZWIERZYNA M, DAVIES M, HINGORANI AD, HUNTER J. CLINICAL TRIAL DESIGN AND DISSEMINATION: COMPREHENSIVE ANALYSIS OF CLINICALTRIALS.GOV AND PUBMED DATA SINCE 2005. BMJ. 2018;361:K2130.
- JOHNSTON BC, VOHRA S. INVESTIGATOR-INITIATED TRIALS ARE MORE IMPARTIAL. NATURE. 2006;443(7108):144.
- GETZ KA, VOGEL JR. SUCCESSFUL OUTSOURCING: TRACKING GLOBAL CRO USAGE. APPLIED CLINICAL TRIALS. 2009;18(6):42.
- HENDERSON L. PHARMA CHOOSES CROS/SERVICES BASED ON NEEDS. APPLIED CLINICAL TRIALS. 2012;21(6):10.

Leave A Comment