How to facilitate the documenting process in clinical trials
Do you have colleagues and employees who consider documenting a time consuming and demanding activity? Why does it happen and which measures can be taken to change this perception?
We work in clinical research, an environment which is necessarily high-regulated. However, we often forget how much effort it took to establish regulations for clinical trials and market approval.
Let´s look back to the 1961–1962, when the Contergan sleeping pills prescribed to pregnant women were found out to cause limbs malformations to unborn children. Or even further in time, when cruel experiments were carried out during the 2nd World War or when in 1937 the untested “medicine” formulation of sulfanilamide and the toxic additive diethylene glycol led to the death of 107 people in USA.
These and other tragical events from the past created over time the awareness to control and regulate more and more clinical research, to reduce the risk that the history will be repeated. The Good Clinical Practices (GCP) were therefore created, allowing to develop standards focused also on ethical and technical aspects and to guarantee the safety of participants and the integrity of data.
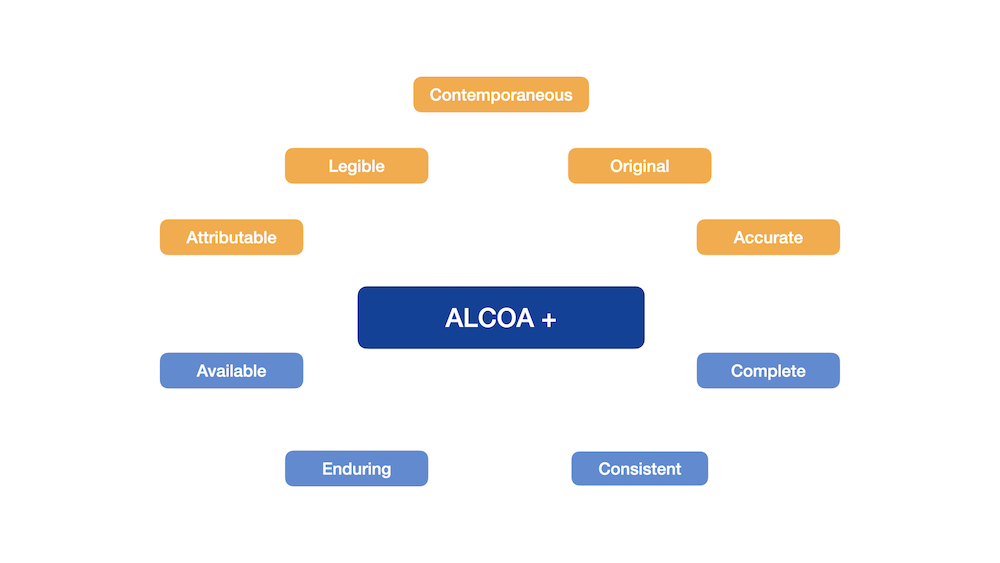
Furthermore, the process to develop a molecule into a final product ready for the market is expensive, time-consuming and it entails many required steps, each of which must be traceable. This is why documenting each process step in a complete manner can ensure the needed transparency, for which the ALCOA and ALCOA plus principle have been established and proven in practice.
The documentation of each project starts with the appropriate agreements to define tasks and responsibilities, the design, the data type and how to collect them, the statistical methods to use and the relevant project management. The next steps cover the tasks for the clinical study/investigations in the clinical site, the data management, the statistic evaluation and the writing of a final report.
Audits/inspections will then verify if the documentation is available and if the requirements are fulfilled as per the applicable regulations.
As Quality Manager I see myself responsible not only for transmitting information and regulatory requirements, but also for raising awareness between employees. In this sense, it is particularly important to transfer the reasons behind the intensive documenting process.
Therefore, a person able to understand this effort and to internalise the fact that subjects´ safety and data integrity are at the forefront, won´t question the effort itself and will be ready to implement the documenting process in an adequate manner.
It´s for this purpose that each year at ICRC-Weyer we attend a GCP Training, which mainly focuses on ethical aspects as those covered by the Declaration of Helsinki, the GCP principles of ICH E6 and DIN EN ISO 14155 as well as further clinical research requirements and responsibilities like the ALCOA+ principle.
Maria Schulz, Quality Manager and Author
Viviana Lerario, Technical Editor

Leave A Comment