An overview of managing safety concerns in the DSURs, RMPs and PBRERs
What are safety concerns?
ICH defines safety concern as an important identified risk, important potential risk or missing information (GVP Annex IV, ICH-E2C(R2) Guideline).
An important identified and potential risk could have an impact on the risk-benefit balance of the product or have implications for public health. What constitutes an important risk will depend upon several factors, including the impact on the individual, the seriousness of the risk and the impact on public health. Normally, any risk that is likely to be included in the contraindications or warnings and precautions section of the product information should be considered important.
What constitutes an identified and potential risk?
An untoward occurrence for which there is adequate evidence of an association with the medicinal product of interest is an identified risk. Evidence may include the following:
- an adverse reaction adequately demonstrated in non-clinical studies and confirmed by clinical data,
- an adverse reaction observed in well-designed clinical trials or epidemiological studies for which the magnitude of the difference, compared with the comparator group on a parameter of interest suggests a causal relationship,
- an adverse reaction suggested by a number of well-documented spontaneous reports where causality is strongly supported by temporal relationship and biological plausibility, such as anaphylactic reactions or application site reactions
An untoward occurrence for which there is some basis for suspicion of an association with the medicinal product of interest but where this association has not been confirmed (ICH-E2F Guideline) constitutes a potential risk. Evidence may include the following:
- non-clinical toxicological findings that have not been observed or resolved in clinical studies;
- adverse events observed in clinical trials or epidemiological studies for which the magnitude of the difference, compared with the comparator group (placebo or active substance, or unexposed group), on the parameter of interest raises a suspicion of, but is not large enough to suggest, a causal relationship;
- a signal arising from a spontaneous adverse reaction reporting system
- an event known to be associated with other active substances within the same class or which could be expected to occur based on the properties of the medicinal product (based on ICH-E2F Guideline)
Gaps in knowledge about a medicinal product, related to safety or use in particular patient populations, which could be clinically significant constitutes missing information.
How are safety concerns presented in pharmacovigilance documents?
After the first subject is exposed to the investigational drug, safety concerns are introduced in a Development Safety Update report (DSUR). As more subjects participate in clinical trials, safety data is accumulated. The safety profile of the investigational drug becomes clearer and the safety concerns for the drug may be defined. Thus, during the drug development and much before the approval of the drug, more knowledge on the important identified and potential risks for the drug may become evident through the annual DSURs.
Gradually, for the approval of the drug, a Risk Management Plan (RMP) will have to be submitted to the regulatory agencies. By this stage, substantial knowledge about the safety concerns is available from the pre-clinical and clinical development. In addition to the important identified and potential risks, the marketing authorization holder (MAH) might also include missing information backed by a scientific rationale. These safety concerns are likely to have an impact on the benefit-risk balance of the drug.
In addition to defining the safety concerns as in the DSUR, an RMP must also include a plan on evaluation of safety concerns after approval of the drug, including the minimization measures. RMPs are updated as and when substantial information on the safety concerns becomes available.
The safety profile of a drug is cemented by the post-marketing experience through the Periodic Benefit-Risk Evaluation Report (PBRER). In the PBRER, the MAH evaluates the safety concerns in context to the benefit-risk analysis. For the drug to stay in the market, the benefits should always out-weigh the risks. This analysis of safety concerns may be periodic and/or cumulative.
It is important to ensure that all safety documents are consistent and present similar information about the safety concerns.
When a signal arising from a spontaneous adverse reaction reporting system indicates strong relationship/basis, or when clear evidence of a class effect appears, an important potential risk may be characterized as an important identified risk. On the contrary, when the safety profile is well characterized for the drug and there is no further impact on the benefit-risk balance, the risks may even be downgraded and removed from the RMP altogether. However, depending on the reporting interval of the report, the MAH can still retain the risk in the PBRER in the summary of safety concerns but propose a rationale to delete the same in the subsequent PBRER in the section on characterization of that respective risk. Thus, a PBRER is a pivotal document to communicate the changes in the safety concerns of a drug.
Over the years, when there would be no trigger to update the RMP, the data presented in the RMP would almost always remain outdated. The PBRER continues to present the periodic and cumulative up-to date evaluations of the safety concerns. An addendum to the Clinical Overview would later communicate the agreed company position of the safety profile of the drug from the PBRER during subsequent renewal submissions.
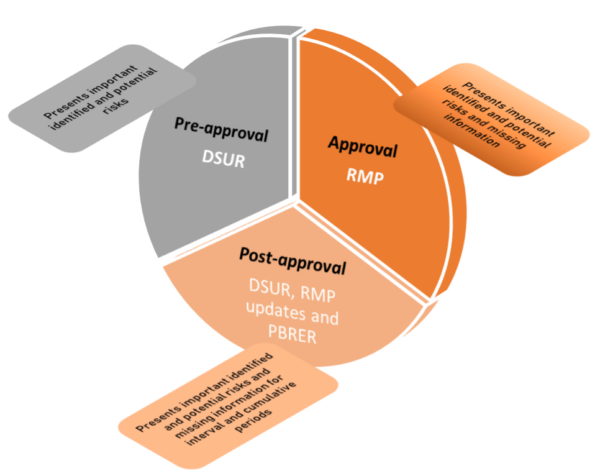
Legend:
DSUR = Development safety update report
RMP = Risk Management Plan
PBRER = Periodic Benefit-Risk Evaluation Report
Having a global submission strategy for RMPs and PSURs may be advantageous for MAHs as less resources may be needed eventually to manage consistency and compliance to communicate the safety concerns to health agencies. As long as there is an on-going patient in a clinical study, the DSURs are prepared and submitted to the regulatory agencies.
ICRC-Weyer GmbH has extensively supported several submissions and challenging projects across different therapeutic areas. ICRC-Weyer GmbH specializes in safety documents and has experienced medical writers handling different types of safety documents. Reach out to us for more information at: info@icrc-weyer.com
Keerthika Gogula, Senior Medical Writer

Leave A Comment