Our Experience
ICRC-Weyer has a sophisticated team of experts with both young and motivated employees and long-term members with immense experience in our field of business. This mixture paired with quality focus and continuous team training is reflected in a large number of successfully finalized projects. We provide consulting on specific scientific questions and cover either individual functional service for clinical trials or full service and rescue support of clinical trials and beyond for our clients.

30 Years of Experience

968 Projects realized

97 Risk Management Plans
Study Phases
ICRC-Weyer supports clinical trials of phase I‑IV, from First-in-Human up to global phase III trials. We are also active in non-interventional and post-marketing studies, investigator-initiated trials of all types and non-trial related projects. Based on this experience we can cover client needs in EDC data collection, data management and biostatistics for every study, be it an early phase or proof of concept trial for a small start-up biotech company or submission support for big pharma companies.

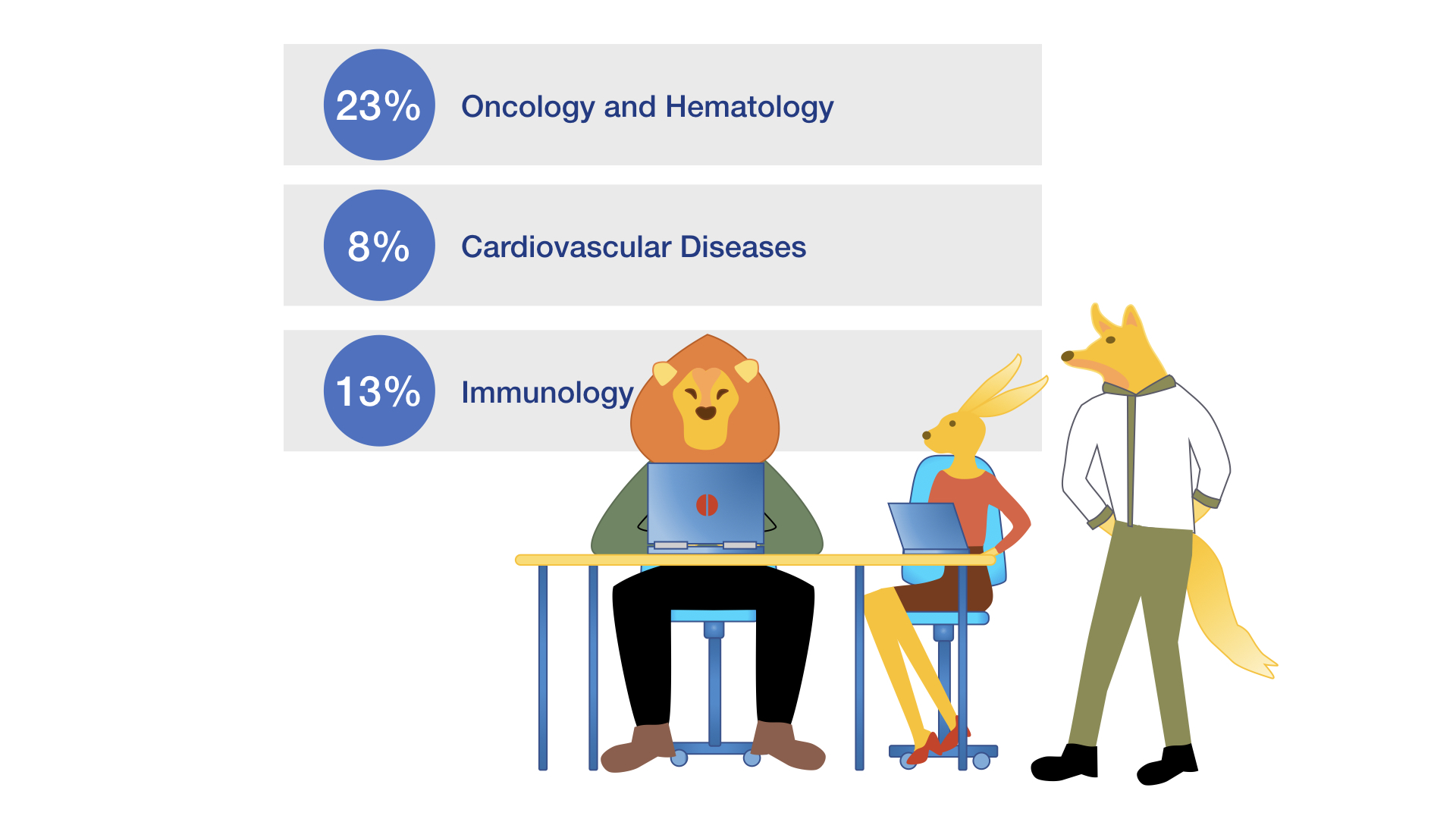
Indications
ICRC-Weyer is experienced in a wide range of different indications with extended expertise in areas like Oncology, Haematology, Immunology, Cardiovascular diseases, Neurodegeneration and Neuroinflammatory diseases. Please feel free to ask for support for your specific indication.
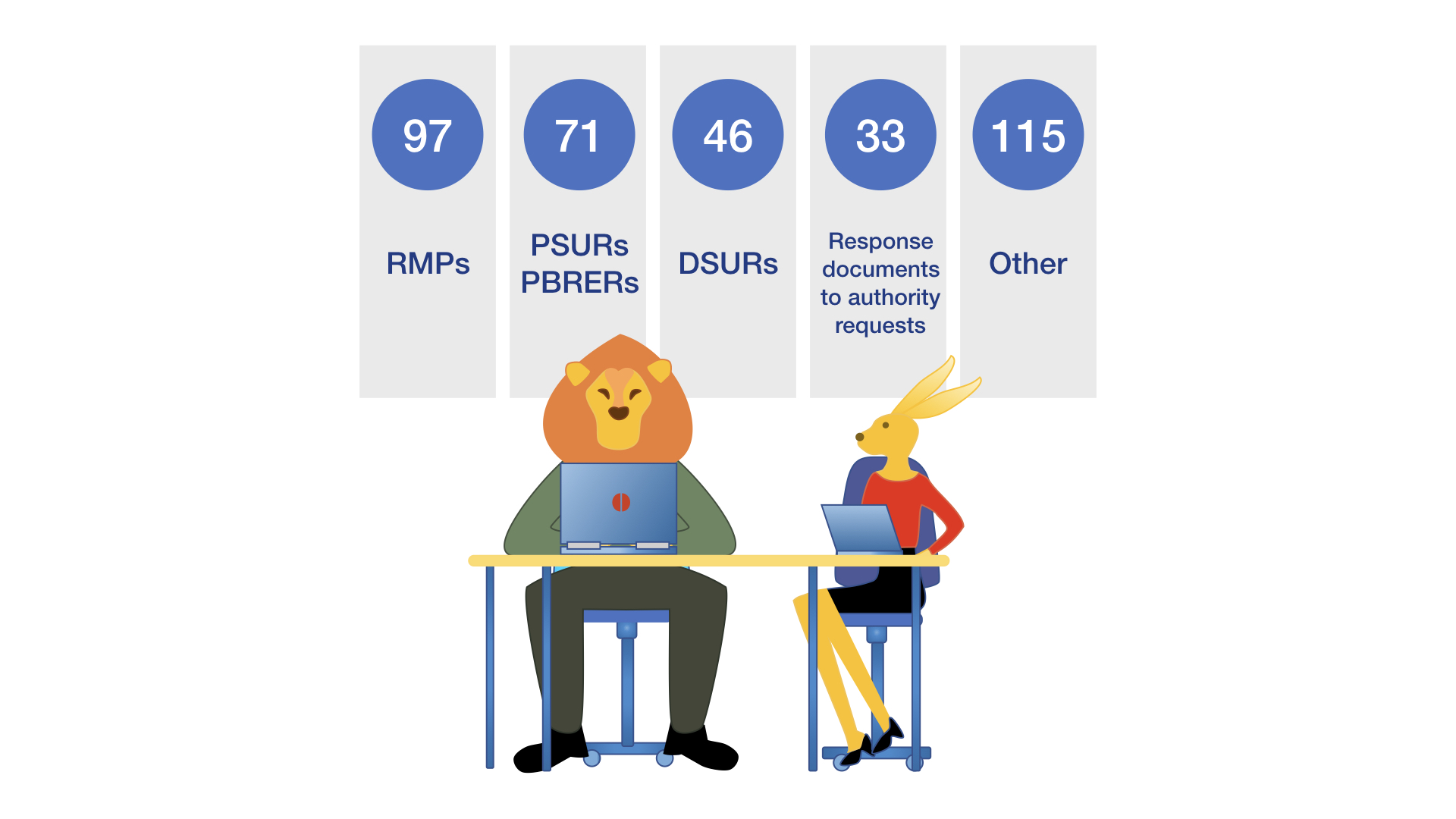
Safety Writing
ICRC-Weyer has considerable experience in the specialized area of safety writing concerning the full range of different pharmacovigilance documents, e.g. RMPs, PSURs/PBRERs and DSURs. Clients value this special aspect of our work in close interaction with our experts.

Medical Review
For Medical Review, ICRC-Weyer developed a system of metrics centered on the number and ratio of medical queries which can be positively resolved.
